ENERGY
Fuel Cell
A fuel cell is an electrochemical cell that converts a source fuel into an electric current. It generates electricity inside a cell through reactions between a fuel and an oxidant (e.g. oxygen) in the presence of an electrolyte. It is primarily the reverse reaction of the electrolysis of water (i.e. H2 + 1/2O2 → H2O + electricity)
Fuel cell of hydrogen & oxygen
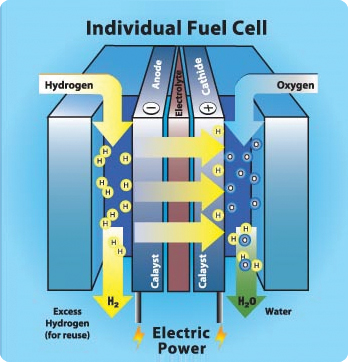
Fuel cells come in many varieties; however, they all work in the same general manner. They are made up of three segments which are sandwiched together: the anode, the electrolyte, and the cathode. At the anode, a catalyst oxidises the fuel (usually hydrogen), turning the fuel into a positively charged ion and a negatively charged electron as electric current. The ions travel through the electrolyte to the cathode, react with oxygen to create water.
The fuel cell can be used to power vehicles or boats even naval vessels such as submarine. As it consumes hydrogen from abundant source such as water with virtually no emission except oxygen and electric current, fuel cell is considered as an environmentally-friendly power source. This is a technology under intensive development worldwide.